| Back Futurehealth | |||||||
|
Original Content at https://www.futurehealth.org/articles/The-Inflammatory-Theory-of-by-Lewis-Mehl-Madrona-120801-604.html |
|||||||
August 1, 2012
The Inflammatory Theory of Depression
By Lewis Mehl-Madrona
In this article, I describe a way of thinking about depression that makes sense of how we collapse from too much stress and from unremitting anxiety and misery. In this theory, eventually life overwhelms our capacity to resist inflammation and it runs away. From August 16th through the 19th, catch me in Hartford, Connecticut, to further discuss these ideas. For details, see
::::::::
In my last blog, I promised to visit the inflammatory theory of depression1, and so I am. When we have a flu or an infection or we are stressed, our immune cells produce molecules (pro-inflammatory cytokines) to help us fight if off. While these signaling molecules activate our immune system, they also act upon the brain, making us feel sick. When our immune systems continues to be activated for long periods of time, such as occurs during chronic stress, the effects of cytokines on the brain can worsen this feeling of sickness and lead to the symptoms of depression.. Inflammation may be an important way in which life's overwhelming events and unresolvable stresses culminate in depression.
Anyone who has experienced a viral or bacterial infection knows what it means to feel sick. Sick people often feel feverish and nauseated, ignore food and beverages, and lose interest in their surroundings and in other people. They tire easily and their sleep is often disturbed. They lose interest in activities and become irritable, and can experience difficulty thinking, including problems with attention and memory. When we are acutely ill, we usually try to ignore these symptoms, knowing that our illness will pass and the symptoms will disappear.
Feeling sick is a normal response to infection, just as being afraid is a normal response to seeing a bear with two cubs ahead of us on the hiking path. The molecules that mediate inflammation and make us feel sick are known as pro-inflammatory cytokines, and include such colorful characters as interleukin-1 α (alpha) and β (beta, usually shortened to IL-1 α and IL-1 β ), tumor necrosis factor- α (TNF- α ) and interleukin-6 (IL-6). It's their effect on the brain that makes us feel sick, which may paradoxically help us to recover (at least from acute illnesses) by putting us into a state of rest and repair. Exposure to these pro-inflammatory cytokines on a long-term basis triggers the development of depression in vulnerable individuals.
How the brain monitors immunity
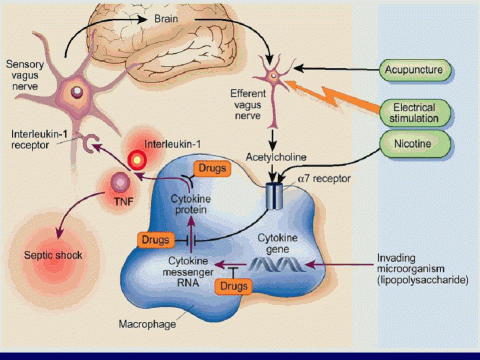
The brain monitors our immune responses by several means. One pathway involves the nerves traveling to it from all parts of the body. Locally produced cytokines activate nerves, such as the vagal nerves, for example, for abdominal infections3,4 and the trigeminal nerves during throat or dental infections.5 These nerves provide the brain with information about what's happening to the organs to which they are attached.
In a second pathway which works through the blood stream, receptors (called TLRs or "Toll-like Receptors") on immune cells (types of macrophages) residing in particular organs around the brain (circumventricular organs and the choroid plexus) respond to pathogen-associated molecules or to high levels of stress hormones by producing pro-inflammatory cytokines.6 These cytokines passively diffuse into the brain7 to produce inflammation of the brain itself.
In a third pathway, transporter molecules carry cytokines across the blood--brain barrier: pro-inflammatory cytokines overflowing in the blood circulation can gain access to the brain through these transport systems8.
A fourth pathway involves cytokine (interleukin-1) receptors that are located on immune cells (macrophages) around blood vessels and on the cells that form the inner lining of veins in the brain. Activation of these receptors by circulating cytokines results in the local production of a pro-inflammatory molecule called prostaglandin E2. This may be how heart disease and stroke happen -- through chronic inflammation of the inside of blood vessels.
Engagement of these immune-to-brain communication pathways ultimately leads to the production of pro-inflammatory cytokines by the glial cells in the brain which surround the neurons. In these ways the brain forms an "image' of what the peripheral immune system is doing. The main difference is that this brain image does not involve an invasion of immune cells into the brain itself and is not distorted by tissue damage that occurs at the site of infection.
The brain circuitry that mediates how cytokines make us feel sick, remains elusive. Cytokine receptors were first localized in the granule cell layer of the dentate gyrus (a part of the cerebral cortex), the pyramidal cell layer of the hippocampus (which converts short term to long term memory) and the anterior pituitary gland. More recently, they were identified in the cells lining veins throughout the brain, in the preoptic and supraoptic areas of the hypothalamus and the sub-fornical organ, and, in lower concentrations, in the paraventricular hypothalamus, cortex, nucleus of the solitary tract, and ventrolateral medulla.
Inflammation and its Controls by University of Pittsburgh
mation and its Controls (from Univ. of Pittsburgh educational material)
See http://www.pitt.edu/~super1/lecture/lec14081/030.htm
Cytokines and sickness behaviour
In general, animals injected with pro-inflammatory cytokines stay in a corner of their cage in a hunched posture and show little or no interest in their physical and social environment unless they are stimulated. They often have increased pain sensitivity.
Anti-inflammatory cytokines work in the opposite direction and help regulate the intensity and duration of how sick we feel probably by inhibiting the production of the pro-inflammatory cytokines and weakening their effects. In particular, injecting anti-inflammatory cytokines like Interleukin-10 or insulin-like growth factor I (IGF-I) into the brains of animals reduces sickness behavior. This is consistent with the idea that in the brain, as in the body's other organs, the natural balance between pro- and anti-inflammatory cytokines regulates the intensity and duration of the immune response.
Aging is associated with increased activity of the immune system, which at the brain level translates into an enhanced production of pro-inflammatory cytokines and a decreased production of anti-inflammatory cytokines. People and animals with type 2 diabetes respond to illness or stress with an increased production of pro-inflammatory molecules and a lowered production of anti-inflammatory molecules.
A role for cytokines in depression?
The similarity between the symptoms of cytokine-induced sickness and depression is striking: in both cases there is a withdrawal from the physical environment and from other people that is accompanied by pain, malaise and decreased reactivity to reward (anhedonia). Moreover, some components of sickness behavior in animals, such as a decreased preference for sweet solutions and reduced social exploration, are improved by antidepressant treatment. In humans, major depression develops in roughly a third of patients who are treated with the cytokines for cancer or hepatitis. In agreement with these findings, depression is more prevalent in people suffering with conditions that lead to chronic inflammation (such as heart diseases, type 2 diabetes and rheumatoid arthritis. Depression could represent a maladaptive version of cytokine-induced sickness, which could occur when activation of the immune response is exacerbated in intensity and/or duration, or that takes place in the context of an increased vulnerability to depression, for example, in individuals who produce excess cortisol. Of course, chronic stress has all these effects. Pro-inflammatory cytokines cause various clinical aspects of depression, including hyperactivity of the hypothalamus--pituitary--adrenal axis (the source of the stress response), disturbed serotonin metabolism, and the symptoms of reduced activity, libido, appetite, movement, etc.
Vegetative, somatic and psychological symptoms of depression
Investigation of the symptoms that developed in cancer and hepatitis C patients receiving treatment with cytokines confirmed that they were caused by the treatment, and revealed that they fell into two distinct categories: early-onset symptoms of depression, which all patients display and which include flu-like symptoms, fatigue, anorexia, pain and sleep disorders, and late-onset psychological symptoms of depression that are experienced by up to half of patients and include mild impairments in thinking and in memory and depressed mood, sometimes accompanied by anxiety and irritability. Pretreatment with paroxetine, an antidepressant, reduces the psychological symptoms but has little or no effect on the early onset symptoms. The patients who developed psychological symptoms of depression scored higher on a depression scale before cytokine treatment was started and had an enhanced pituitary--adrenal response following the first injection of cytokines, indicating that vulnerability to immunotherapy-induced depression involves both psychological and physiological risk factors.
A role for tryptophan?
Treatment with cytokines alters the biochemistry of patients; the most revealing sign is a pronounced reduction in blood levels of tryptophan, which correlates with the patients' depression scores 3 weeks into the treatment. Tryptophan is an essential amino acid that is actively transported into the brain to make serotonin. The availability of tryptophan determines the rate of serotonin synthesis in the brain. Acute tryptophan depletion decreases mood in vulnerable people who have a familial history of major depressive disorders or are drug-free in remission after an episode of major depression.
Alternative mechanisms for cytokine-induced depression
There is evidence that cytokines might modulate serotonin neurotransmission by mechanisms other than the decrease in tryptophan levels. A hyperactive hypothalamus--pituitary--adrenal axis is often associated with depression. Pro-inflammatory cytokines acutely and potently activate the hypothalamus--pituitary--adrenal axis. In conditions of chronic inflammation, pro-inflammatory cytokines can cause receptor resistance to stress hormones. In addition, pro-inflammatory cytokines seem to promote expression of a form of the glucocorticoid (stress hormone) receptor that is inactive but still binds stress hormones. At the hypothalamic level, this cytokine-dependent glucocorticoid receptor resistance can explain the reduced ability of high levels of stress hormones to shut down their own production (through turning off the production of corticotrophin releasing factor or CRF). At the level of the immune cells, the normally inhibitory effect of stress hormones on further cytokine production and action would no longer work, setting the stage for a "run-away freight train effect" that would result in an ever increasing production of pro-inflammatory cytokines. This increased inflammatory response in the brain results in a decreased inhibitory feedback on CRF by the stress hormones, thereby ever intensifying the stress-response.
Neuroanatomy of cytokine-induced depression
The search for a possible neuroanatomical basis of cytokine-induced depression has focused on the brain circuits that are involved in emotion processing and psychomotor retardation, both of which are altered in patients with clinical depression. Neuroimaging data of depressed patients show decreased baseline activity in the frontal and temporal cortex and the insula, and increased activity in the cerebellum, subcortical and limbic regions. In agreement with this, cancer patients treated with cytokines display decreased activity in the dorsal prefrontal cortex (which is what occurs in people diagnosed with depression) and increased activity in the cerebellum and basal ganglia (especially in the left putamen and nucleus accumbens where this increased activity is correlated with fatigue and lack of energy. These data These findings are consistent with the greater difficulty of cytokine treated patients to regulate their emotions.
Implications for depression in medically ill people
A growing amount of information points to the importance of the relationship between inflammation and depression in physically ill patients and in conditions that are associated with increased activity of the immune system, including aging and obesity. For instance, the prevalence of depression in patients with coronary heart disease, a disease in which inflammation is now recognized as a major contributing factor, is three times higher than in the general population. Depression has long been known to be a risk factor for subsequent cardiac events and mortality.
Mechanisms of enhanced response to systemic inflammation
Conditions of chronic inflammation exacerbate the sickness and depression-like behaviors that develop in response to acute inflammation. This phenomenon can be seen in mice models of degenerative brain disease, type 2 diabetes, and normal ageing, and might be due to an effect called "priming'. For example, in mice that have previously been exposed to pro-inflammatory cytokines (the priming stimulus), exposure to a triggering stimulus, such as an additional acute stress or an infection, leads to an exaggerated production of pro-inflammatory cytokines.
Future directions
The symptoms of sickness (for example, fatigue, reduced appetite, sleep disorders, altered mood and thought) have a negative impact on the quality of life of patients with chronic inflammatory disorders, but not much can be done to alleviate these symptoms. Activities like physical exercise, hypnosis, and even Reiki could have a beneficial effect on the symptoms of sickness, as could anti-inflammatory diets.
For my facts, I relied extensively on an article by Robert Dantzer*,"-, Jason C. O'Connor*, Gregory G. Freund*,"-, Rodney W. Johnson*, and Keith W. Kelley. From inflammation to sickness and depression: when the immune system subjugates the brain found in Nature Reviews of Neuroscience, January 2008, volume 9(1): 46--56, which provides much more technical information and all the scientific references.
For anyone who wishes to catch up with me, I will be doing "Healing Camp" in Tuscany, Italy, with Barbara Mainguy, from August 5th through the 12th. More information is available on my website at http://www.mehl-madrona.com. Also, from August 16th through the 19th, I will be in Hartford, Connecticut, teaching Cherokee Bodywork. For details, see http://sukhasala.com/workshops-events/#madrona. As with all Coyote Institute events, no one is ever turned away, regardless of inability to pay.
Authors Website: www.mehl-madrona.com
Authors Bio:
Lewis Mehl-Madrona graduated from Stanford University School of Medicine and completed residencies in family medicine and in psychiatry at the University of Vermont. He is the author of Coyote Medicine, Coyote Healing, Coyote Wisdom, and Narrative Medicine.