In my last blog, I promised to visit the inflammatory theory of depression1, and so I am. When we have a flu or an infection or we are stressed, our immune cells produce molecules (pro-inflammatory cytokines) to help us fight if off. While these signaling molecules activate our immune system, they also act upon the brain, making us feel sick. When our immune systems continues to be activated for long periods of time, such as occurs during chronic stress, the effects of cytokines on the brain can worsen this feeling of sickness and lead to the symptoms of depression.. Inflammation may be an important way in which life's overwhelming events and unresolvable stresses culminate in depression.
Anyone who has experienced a viral or bacterial infection knows what it means to feel sick. Sick people often feel feverish and nauseated, ignore food and beverages, and lose interest in their surroundings and in other people. They tire easily and their sleep is often disturbed. They lose interest in activities and become irritable, and can experience difficulty thinking, including problems with attention and memory. When we are acutely ill, we usually try to ignore these symptoms, knowing that our illness will pass and the symptoms will disappear.
Feeling sick is a normal response to infection, just as being afraid is a normal response to seeing a bear with two cubs ahead of us on the hiking path. The molecules that mediate inflammation and make us feel sick are known as pro-inflammatory cytokines, and include such colorful characters as interleukin-1 Î � (alpha) and Î � (beta, usually shortened to IL-1 Î � and IL-1 Î � ), tumor necrosis factor- Î � (TNF- Î � ) and interleukin-6 (IL-6). It's their effect on the brain that makes us feel sick, which may paradoxically help us to recover (at least from acute illnesses) by putting us into a state of rest and repair. Exposure to these pro-inflammatory cytokines on a long-term basis triggers the development of depression in vulnerable individuals.
How the brain monitors immunity
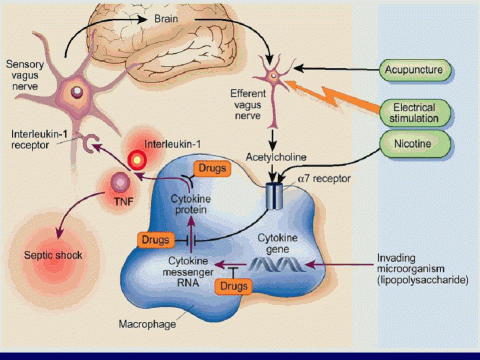
The brain monitors our immune responses by several means. One pathway involves the nerves traveling to it from all parts of the body. Locally produced cytokines activate nerves, such as the vagal nerves, for example, for abdominal infections3,4 and the trigeminal nerves during throat or dental infections.5 These nerves provide the brain with information about what's happening to the organs to which they are attached.
In a second pathway which works through the blood stream, receptors (called TLRs or "Toll-like Receptors") on immune cells (types of macrophages) residing in particular organs around the brain (circumventricular organs and the choroid plexus) respond to pathogen-associated molecules or to high levels of stress hormones by producing pro-inflammatory cytokines.6 These cytokines passively diffuse into the brain7 to produce inflammation of the brain itself.
In a third pathway, transporter molecules carry cytokines across the blood--brain barrier: pro-inflammatory cytokines overflowing in the blood circulation can gain access to the brain through these transport systems8.
A fourth pathway involves cytokine (interleukin-1) receptors that are located on immune cells (macrophages) around blood vessels and on the cells that form the inner lining of veins in the brain. Activation of these receptors by circulating cytokines results in the local production of a pro-inflammatory molecule called prostaglandin E2. This may be how heart disease and stroke happen -- through chronic inflammation of the inside of blood vessels.
Engagement of these immune-to-brain communication pathways ultimately leads to the production of pro-inflammatory cytokines by the glial cells in the brain which surround the neurons. In these ways the brain forms an "image' of what the peripheral immune system is doing. The main difference is that this brain image does not involve an invasion of immune cells into the brain itself and is not distorted by tissue damage that occurs at the site of infection.
The brain circuitry that mediates how cytokines make us feel sick, remains elusive. Cytokine receptors were first localized in the granule cell layer of the dentate gyrus (a part of the cerebral cortex), the pyramidal cell layer of the hippocampus (which converts short term to long term memory) and the anterior pituitary gland. More recently, they were identified in the cells lining veins throughout the brain, in the preoptic and supraoptic areas of the hypothalamus and the sub-fornical organ, and, in lower concentrations, in the paraventricular hypothalamus, cortex, nucleus of the solitary tract, and ventrolateral medulla.
Inflammation and its Controls by University of Pittsburgh
mation and its Controls (from Univ. of Pittsburgh educational material)
See http://www.pitt.edu/~super1/lecture/lec14081/030.htm
Cytokines and sickness behaviour
In general, animals injected with pro-inflammatory cytokines stay in a corner of their cage in a hunched posture and show little or no interest in their physical and social environment unless they are stimulated. They often have increased pain sensitivity.
Anti-inflammatory cytokines work in the opposite direction and help regulate the intensity and duration of how sick we feel probably by inhibiting the production of the pro-inflammatory cytokines and weakening their effects. In particular, injecting anti-inflammatory cytokines like Interleukin-10 or insulin-like growth factor I (IGF-I) into the brains of animals reduces sickness behavior. This is consistent with the idea that in the brain, as in the body's other organs, the natural balance between pro- and anti-inflammatory cytokines regulates the intensity and duration of the immune response.
Aging is associated with increased activity of the immune system, which at the brain level translates into an enhanced production of pro-inflammatory cytokines and a decreased production of anti-inflammatory cytokines. People and animals with type 2 diabetes respond to illness or stress with an increased production of pro-inflammatory molecules and a lowered production of anti-inflammatory molecules.